Drawing the Lewis Structure for BeCl2
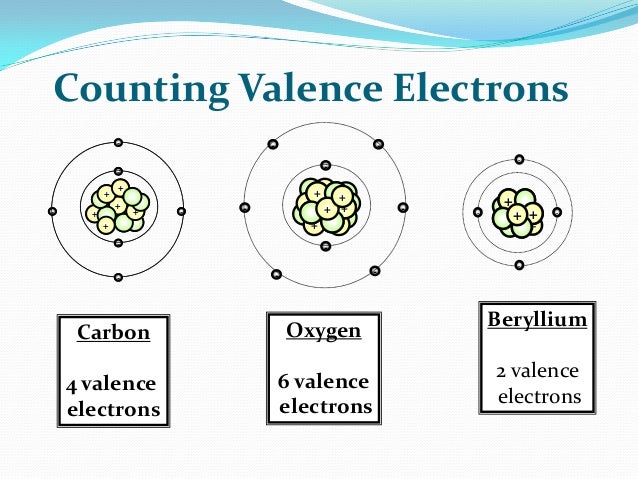
Beryllium has a total of 4 electrons when neutral. Beryllium has 2 electron shells. In order to maintain a stable 'happy' atom, it will do anything to get rid of the 2 outer electrons, leaving 2 left. Remember, the innermost electron shell can hold up to 2 electrons. And the rest, 8 according to the octet rule. Spamblocking software programs download free. Beryllium has two valence electrons. Valence electrons are those electrons that are capable of participating in the formation of chemical bonds with other atoms. These chemical bonds occur through the sharing of these two electrons with other atoms. Valence electrons get their name from their location within an atom.
Viewing Notes:
- The BeCl2 Lewis structure is similar to BeF2 since F is in Group 7 and has 7 valence electrons.
- Beryllium (Be) doesn't need 8 valence electrons to have an octet (Be often only needs 4).
- If you're not sure you have the best Lewis structure for BeCl2 you can calculate the formal charges. You'll find the Be in BeCl2 only has 4 valence electrons.
- For the BeCl2 Lewis structure there are a total of 16 valence electrons available.



See the Big List of Lewis Structures
Transcript: Hi, this is Dr. B. Let's do the Lewis structure for Beryllium Chloride. On the periodic table Beryllium is in group 2, it has 2 valence electrons; Chlorine--group 7--7, but we have 2 of those Chlorines. We multiply that by 2 and add this, we get 16 total valence electrons. Let's draw it. We'll put the least electronegative, Beryllium, at the center, and on either side we'll put a Chlorine. And we have 16 valence electrons. Put 2 between the atoms to form the bonds, and then around the outside atoms, 6, 8, 10, 12, 14, 16.
We've used up all the valence electrons and let's see if we have octets. This is good on this Chlorine, and over here we're fine. We have 8 on both. But the Beryllium in the center only has 4. I know that Beryllium is kind of an exception. It doesn't necessarily need 8 valence electrons.
So I'm not sure if this is the right structure. The way to check is to use formal charges. So we have an equation here to calculate the formal charge for each of the atoms. So let's start with this Chlorine right here. Chlorine's in grou 7, so it has 7 valence elecrons. Nonbonding, these ones right here, there are 6 of those. And bonding, there are 2 of those, but we're going to divide that by 2. Seven minus 6 minus 1 is 0. The formal charge on Chlorine is 0, and since both Chlorines are the same, that's going to be 0, too.
Valence Electrons In Sodium
OK, let's check out the Beryllium right here. So Beryllium is in group 2, 2 valence electrons. Nonbonding: well, all of the valence electrons for Beryllium are involved in bonds, so that's 0. Minus bonding, these right here dvided by 2. Two minus 0 minus 2 is 0, so the formal charge on Beryllium is also 0.
Number Of Valence Electrons In Zinc
When the formal charges are all 0, then I'm comfortable that this is really going to be the best structure. This is Dr. B., and thanks for watching. Demo download ds play games.
