- Atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).
- Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
- Atom definition at Dictionary.com, a free online dictionary with pronunciation, synonyms and translation.

Student directions Build an Atom activity- homework version Learning Goals: Students will be able to 1. Make atom models that show stable atoms or ions. Use given information about subatomic particles to Identify an element and its position on the periodic table Draw models of atoms Determine if the model is for a neutral atom or an ion. Predict how addition or subtraction of a proton. An atom is 9% space. The whole mass of an atom is in the nucleus, and between nucleus and the edge of the atom, there are enormous distances. To understand it more if you increase the size of the nucleus to that of a coin the edge of the atom would be at a distance of 2-3 kilometer.
Article- Atomic model
- Basic properties
- The electron
- The nucleus
- Development of atomic theory
- The beginnings of modern atomic theory
- Studies of the properties of atoms
- Models of atomic structure
- Advances in nuclear and subatomic physics
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
An Atom Is Made Of What
Most of the atom is empty space. The rest consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. Electrons are attracted to any positive charge by their electric force; in an atom, electric forces bind the electrons to the nucleus.
Because of the nature of quantum mechanics, no single image has been entirely satisfactory at visualizing the atom’s various characteristics, which thus forces physicists to use complementary pictures of the atom to explain different properties. In some respects, the electrons in an atom behave like particles orbiting the nucleus. In others, the electrons behave like waves frozen in position around the nucleus. Such wave patterns, called orbitals, describe the distribution of individual electrons. The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells.
This article opens with a broad overview of the fundamental properties of the atom and its constituent particles and forces. Following this overview is a historical survey of the most influential concepts about the atom that have been formulated through the centuries. For additional information pertaining to nuclear structure and elementary particles, seesubatomic particles.
Atomic model
Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Attempts to separate these smaller constituent particles require ever-increasing amounts of energy and result in the creation of new subatomic particles, many of which are charged.
As noted in the introduction to this article, an atom consists largely of empty space. The nucleus is the positively charged centre of an atom and contains most of its mass. It is composed of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms. Other subatomic particles may be found in association with these three types of particles. They can be created only with the addition of enormous amounts of energy, however, and are very short-lived.
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10−10 metre. The radius of an atom measures 1–2 Å. Compared with the overall size of the atom, the nucleus is even more minute. It is in the same proportion to the atom as a marble is to a football field. In volume the nucleus takes up only 10−14 metres of the space in the atom—i.e., 1 part in 100,000. A convenient unit of length for measuring nuclear sizes is the femtometre (fm), which equals 10−15 metre. The diameter of a nucleus depends on the number of particles it contains and ranges from about 4 fm for a light nucleus such as carbon to 15 fm for a heavy nucleus such as lead. In spite of the small size of the nucleus, virtually all the mass of the atom is concentrated there. The protons are massive, positively charged particles, whereas the neutrons have no charge and are slightly more massive than the protons. The fact that nuclei can have anywhere from 1 to nearly 300 protons and neutrons accounts for their wide variation in mass. The lightest nucleus, that of hydrogen, is 1,836 times more massive than an electron, while heavy nuclei are nearly 500,000 times more massive.
An Atom Is Mostly Empty Space
Basic properties
Atomic number
The single most important characteristic of an atom is its atomic number (usually denoted by the letter Z), which is defined as the number of units of positive charge (protons) in the nucleus. For example, if an atom has a Z of 6, it is carbon, while a Z of 92 corresponds to uranium. A neutral atom has an equal number of protons and electrons so that the positive and negative charges exactly balance. Since it is the electrons that determine how one atom interacts with another, in the end it is the number of protons in the nucleus that determines the chemical properties of an atom.

- key people
- related topics
The key difference between cell and atom is that acell is made of molecules whereas atoms make up molecules.

Cells are the smallest functioning unit in a living organism. It contains many macromolecules. Meanwhile, atoms make up these macromolecules. Hence, an atom is the smallest unit of matter. Usually, a cell is on the micrometre scale while an atom is in the angstrom scale.
CONTENTS
1. Overview and Key Difference
2. What is a Cell
3. What is an Atom
4. Side by Side Comparison – Cell vs Atom in Tabular Form
5. Summary
What is a Cell?
A cell is the smallest functioning unit of living organisms. In other words, it is the smallest unit of life. Therefore, we call it the building block of life. There are different types of cells such as microorganisms, animal cells and plant cells. They have different structures. But, all these cells have an outer membrane which holds the content inside.
When considering the structure of a cell, it contains a cytoplasm enclosed with a cell membrane, and the cytoplasm holds different organelles such as mitochondria. Therefore, different macromolecules, such as proteins, nucleic acid, carbohydrates, lipids, etc., make up the cells.
What is an Atom?
Atom is the smallest particle of a chemical element that can exist. Therefore, it is the smallest unit of matter, and a certain atom represents the properties of the chemical element to which it belongs. All gases, solid matter, liquids and plasma contain atoms. These are minute units; typically the size is around 100 picometers.
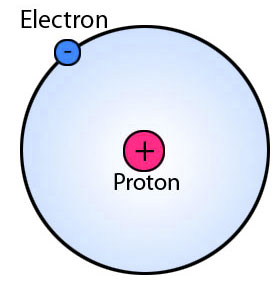
Figure 02: Typical Structure of an Atom
When considering the structure of an atom, it contains a nucleus and electrons moving around the nucleus. Further, protons and neutrons (and there are some other subatomic particles as well) make up the atomic nucleus. Typically, the number of neutrons, protons and electrons are equal to each other, but in the case of isotopes, the number of neutrons is different from that of protons. We call both protons and neutrons “nucleons”.
An Atom Is Matter
Around 99% of the atom’s mass is centred in the nucleus because the mass of an electron is almost negligible. Among these subatomic particles, a proton has +1 charge; an electron has -1 charge and a neutron has no charge. Go ho the starry night download. If the atom has equal numbers of protons and electrons, then the overall charge of the atom is zero; lack of one electron results in a +1 charge and a gain of one electron gives -1 charge to the atom.
What is the Difference Between Cell and Atom?
A cell is a biological unit, while an atom is a chemical unit. Besides, the key difference between cell and atom is that a cell is made of molecules whereas atoms make up molecules. Also, when considering the composition of these units, a typical cell contains cytoplasm, cell membrane, nucleus, etc. while an atom contains small subatomic particles such as electrons, protons and neutrons.
Moreover, a further difference between cell and atom is that a cell contains macromolecules such as proteins, carbohydrates, lipids and nucleic acid while molecules are made of atoms.
Summary – Cell vs Atom
A cell is a biological unit, while an atom is a chemical unit. In summary, the key difference between cell and atom is that a cell is made of molecules whereas atoms make up molecules.
Reference:
1. Cooper, John A., et al. “Cell.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., Available here.
An Atom Is Made Up Of
Image Courtesy:
Nvidia nforce networking controller driver download win7. 1. “Simple diagram of animal cell (en)” By domdomegg – Own work (CC BY 4.0) via Commons Wikimedia
2. “Atom Diagram” By AG Caesar – Own work (CC BY-SA 4.0) via Commons Wikimedia
An Atom Is Electrically Neutral
Related posts:
