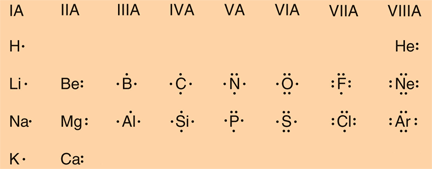
The energy level of an atom's valence electrons correspond to its period or horizontal row on the periodic table. Hydrogen and helium, both in the first period, have their valence electrons in the. Valence Electrons and Ion Formation for the First 20 Elements Element Total Number of Electrons in Neutral Atom Valence Electrons Gain or Lose Electrons Ion Formed Hydrogen 1 1 Gain or Lose 1 H+ or H-Helium 2 2 None None Lithium 3 1 Lose 1 Li+ Beryllium 4 2 Lose 2 Be2+ Boron 5 3 Lose 3 B3+.
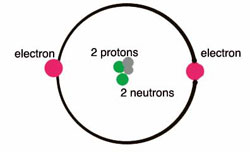
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells.
Learning Objectives
- Construct an atom according to the Bohr model
Key Points
- In the Bohr model of the atom, the nucleus contains the majority of the mass of the atom in its protons and neutrons.
- Orbiting the positively-charged core are the negatively charged electrons, which contribute little in terms of mass, but are electrically equivalent to the protons in the nucleus.
- In most cases, electrons fill the lower- energy orbitals first, followed by the next higher energy orbital until it is full, and so on until all electrons have been placed.
- Atoms tend to be most stable with a full outer shell (one which, after the first, contains 8 electrons), leading to what is commonly called the ” octet rule “.
- The properties of an element are determined by its outermost electrons, or those in the highest energy orbital.
- Atoms that do not have full outer shells will tend to gain or lose electrons, resulting in a full outer shell and, therefore, stability.
Key Terms
- octet rule: A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons. (Hydrogen is excluded because it can hold a maximum of 2 electrons in its valence shell. )
- electron shell: The collective states of all electrons in an atom having the same principal quantum number (visualized as an orbit in which the electrons move).
Electron Shells and the Bohr Model
Share your personnal experiences. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number.

An early model of the atom was developed in 1913 by Danish scientist Niels Bohr (1885–1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol “n.” For example, 1n represents the first energy level located closest to the nucleus.
He Valence Electrons
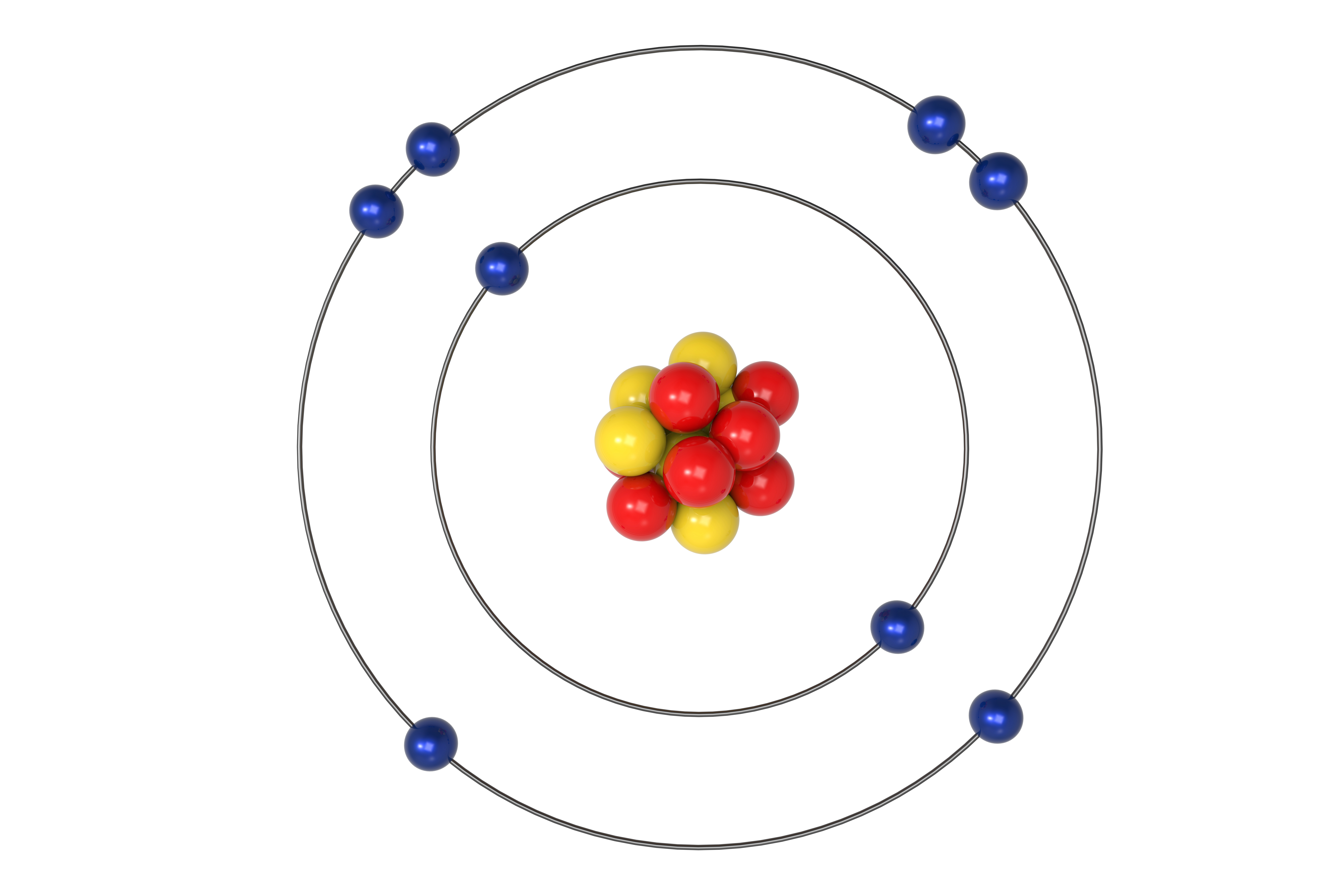
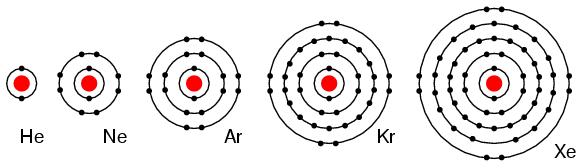
Electrons fill orbit shells in a consistent order. Under standard conditions, atoms fill the inner shells (closer to the nucleus) first, often resulting in a variable number of electrons in the outermost shell. The innermost shell has a maximum of two electrons, but the next two electron shells can each have a maximum of eight electrons. This is known as the octet rule which states that, with the exception of the innermost shell, atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. Examples of some neutral atoms and their electron configurations are shown in. As shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Similarly, neon has a complete outer 2n shell containing eight electrons. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Theoretically, they would be more energetically stable if they followed the octet rule and had eight.
List Of Valence Electrons For Each Element
Number Of Valence Electrons In Helium
An atom may gain or lose electrons to achieve a full valence shell, the most stable electron configuration. The periodic table is arranged in columns and rows based on the number of electrons and where these electrons are located, providing a tool to understand how electrons are distributed in the outer shell of an atom. As shown in, the group 18 atoms helium (He), neon (Ne), and argon (Ar) all have filled outer electron shells, making it unnecessary for them to gain or lose electrons to attain stability; they are highly stable as single atoms. Their non-reactivity has resulted in their being named the inert gases (or noble gases). In comparison, the group 1 elements, including hydrogen (H), lithium (Li), and sodium (Na), all have one electron in their outermost shells. This means that they can achieve a stable configuration and a filled outer shell by donating or losing an electron. As a result of losing a negatively-charged electron, they become positively-charged ions. When an atom loses an electron to become a positively-charged ion, this is indicated by a plus sign after the element symbol; for example, Na+. Group 17 elements, including fluorine and chlorine, have seven electrons in their outermost shells; they tend to fill this shell by gaining an electron from other atoms, making them negatively-charged ions. When an atom gains an electron to become a negatively-charged ion this is indicated by a minus sign after the element symbol; for example, F-. Thus, the columns of the periodic table represent the potential shared state of these elements’ outer electron shells that is responsible for their similar chemical characteristics.
